Cell Based Assay Basics
Intro
Cell-based assays are used as disease models when screening therapies. Plate-based assays using adherent mammalian cells are an industry standard and most adaptable to automation. Let’s explore what a cell-based assay is, how it’s used in drug discovery, and some considerations when adapting a manual cell-based assay protocol to an automated one. Additionally, I’d like to introduce you to the Biotek Multiflo FX, which is a workhorse robot when it comes to cell-based assays.
What is a cell-based assay?

A cell-based assay is a tool used to simulate a disease state or cellular mechanism which can measure the safety and biological function of a therapy in a preclinical context. They can use human or animal cells, often mammalian, and a signal is measured to indicate the efficacy of the therapy being tested. This can be done in various plate formats from 4 well down to 1536. Signal is detected by specialized reagents in an assay.
Some reagents can measure cellular byproducts of wildtype cells and viruses, or cell lines and viruses can be engineered to express proteins like luciferase or gfp when certain conditions are met. Some common assay readouts are:
Luminescence: Luminescence refers to a process where light is released. Two common types of luminescence in assays are chemiluminescence like the chemical reaction from CTG, which binds ATP to produce a signal, and Bioluminescence like Firefly Luciferase.
Fluorescence: Fluorescence is a form of luminescence where light is absorbed at a particular wavelength and emitted at a different one. Recall how color is perceived at different wavelengths on the electromagnetic spectrum. Some common fluorescent readouts are: GFP(green fluorescent protein) R(ed)FP, Halotag, Snaptag, and Clip
Imaging: Imaging uses microscopy technology to take an image and detect things like fluorescence, staining, digital phase contrast, and brightfield capture to name a few. I’m planning an entire post on imaging in the near future, so hang tight for a deep dive on that. The main point I want to emphasize here is that you can run imaging protocols on 384 and 1536 well plates to get detailed data at a small scale!
How Cell Based Assays are Used in Drug Discovery
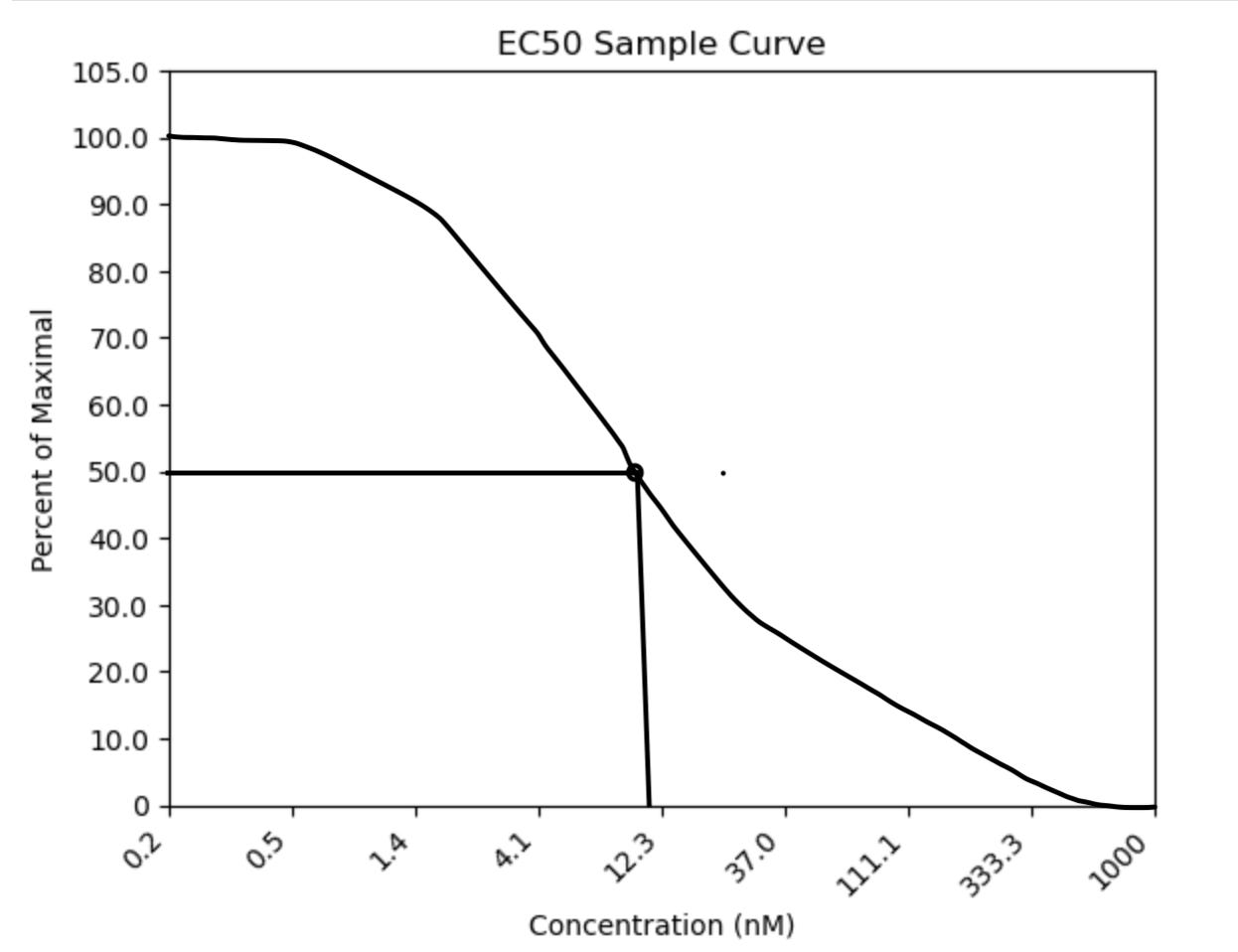
Potency data comes in the form of an EC50 or IC50 curve. This curve gives you an EC50 value by testing the dose-response of a therapy in your disease model. This value at its core is measuring the concentration where there is 50% of the maximal “effect” of your treatment. If your “effect” is inhibition, IC50 is the more accurate term, but it can still be referred to as the EC50. The goal of a screening effort is to lower your EC50 value which means your treatment is becoming more potent with each iteration. Lower EC50 values roughly translate to needing less drug to have an effect, which can save money in the long run.
In this figure, we see a 3x titration of drug starting from 1000nM of compound. In this sample assay, the presence of signal indicates no activity in the assay. Imagine a scenario where the presence of signal equals the presence of virus. 100% suppression of the virus will cause 0% signal and vice versa. We see that at 50% of maximal signal, the concentration of drug is around 10nM or EC50 = 10nM. This means that 10nM of drug is needed to suppress the virus signal 50%. I made the blank plot for this graph using Python! I drew the actual curve in. I’m still working on graphing the actual data, so keep an eye out for when I figure that out.
Aside from potency, you can measure cytotoxicity, biochemical mechanisms, and off-target interactions with a cell-based assay. While there is a tendency to focus on potency data in early research, you need a comprehensive data package to ensure that your therapies won’t fail in more complex biological environments. Full disclosure, I’ve mostly worked on potency assays, so I can’t speak too much on other cell-based assay measurements. Cell-based assays are typically the precursor to in-vivo work because they can be more biologically relevant than biochemical assays but often lack the complexity of interactions that occur in an in vivo disease model. Additionally, the small reaction volume and ease of cell culture versus animal husbandry means you can test more material in vitro before testing your top hits in vivo.
Considerations for Automation
As this is an automation blog, here are some things to take into consideration when using automation to set up your cell-based assays. Let’s start with cell culture Cell culture:
Consider generating a large cell bank at the start of a screening effort to ensure that cells are of similar quality when being used for an assay.
Test the effects of passage number in your assay to find the optimal passaging window for your cells to give the best signal for your assay.
Minimize cell passages from thaw to reduce user error, but be mindful that freshly thawed cells may grow slower and produce weaker signals, so analyze the trade-offs before implementing this approach
It’s possible to run an assay with freshly thawed cells. You’ll maximize interoperability, but could sacrifice signal and data quality.
Cell seeding density is crucial to your disease model operating correctly. When choosing seeding density in a plate format, take into consideration the growth rate of the cells to avoid cell overgrowth
Dispensing cells with automation can increase interoperability due to the advantages of automation I’ve highlighted in previous posts. Two modes of automated cell dispense I have used are the FCA and MCA of the Tecan Fluent, which use air displacement for their pipetting actions, or the peristaltic pump from the Biotek Multiflo FX. Optimal dispensing using these modes will depend on many factors including cell size, speed of dispense, clumping, sterility, and cells settling:
Cell size: Eukaryotic cells have an average diameter of 25µm, so the bore size could be a factor when dispensing cells, especially when using clumpier cell lines.
Speed of dispense: As mentioned before, the speed at which you aspirate and dispense cells can be controlled on a robot. While using faster dispensing speed could speed up your assay setup, consider the effect on your cells. Speeds that are too fast could produce enough force to lyse your cells or cause splashing. Test dispensing speeds as part of your assay optimization.
Clumping: Clumpy cells will add variability when counting and dispensing cells. Consider taking some measures before counting your cells, like using a cell strainer or anti-clumping reagent to obtain more accurate cell counts.
Sterility: Using disposable tips on a robot like the Tecan, which is already in its own positive pressure sterile environment, is the most sterile mode of cell dispense. I’ve used the Multiflo FX in a BSC and peristaltic cassettes to dispense cells. When sterilized and maintained properly, the Multiflo FX can dispense cells quickly and with a CV as low as 1%. It’s a good way to save on tips but requires monitoring of your cell culture for contamination from pathogens or other cell lines.
Cells settling: Depending on your throughput, cells can settle at the bottom of your dispensing receptacle. This lowers the density of cells being dispensed from what you counted. One option is to manually shake your container after every few plates for more consistent cell plating density. A walk-away solution is to use an agitator like the Spin Vessel, which will gently mix and suspend your cells.
In the next section, I’ll delve deeper into a liquid dispenser I’m very familiar with: the Biotek Multiflo FX and Biostack.
Multiflo FX and Biostack
The Multiflo FX is a versatile large-volume liquid dispenser that also has plate washing and media exchange capabilities. You can pair it with a Biostack, which can hold up to 18 plates, for walkaway automation or integrate it into a robotic workcell. The Multiflo FX can replace up to five liquid handlers. Its dispense modes include:
Two peristaltic pumps that use re-usable 8 channel cassettes that come in multiple sizes. The cassettes can be sterilized and autoclaved for your cell culture needs.
Two syringe pumps with 16 channel dispense pins can quickly dispense reagents into a 384 or 1536 well plate.
8 Channel plate washing with separate aspirating and dispensing pins. Great for media exchanging your cell culture plates.
The software had one of the most user-friendly user interfaces I have seen in a liquid handler. I could teach someone to program loops for this robot within an hour. Unlike the Tecan Fluent, which has its own hood, the Multiflo FX needs its own BSC when working with cells. It fit into a standard 6-foot BSC
in my last lab. When tested with a Tartrazine absorbance assay, CV for the peristaltic pump dispense was ~2%. You’ll save a ton on tips with the reusable peristaltic pump cassettes that come in two sizes of tubing and 2 bore sizes. They are autoclavable and have introduced little to no cross-contamination between cell lines when decontaminated between runs in my experience.
Conclusion
Cell-based assays are a crucial tool for drug discovery efforts. In this post, we explored the basics of cell-based assays, their significance in drug discovery, and key factors to consider when automating these assays. We also introduced the Biotek Multiflo FX, a valuable tool for streamlining cell-based assay workflows. Next week, I’d like to discuss more complex disease models that are arguably more clinically relevant than the adherent cell-based assays I discussed in this post.